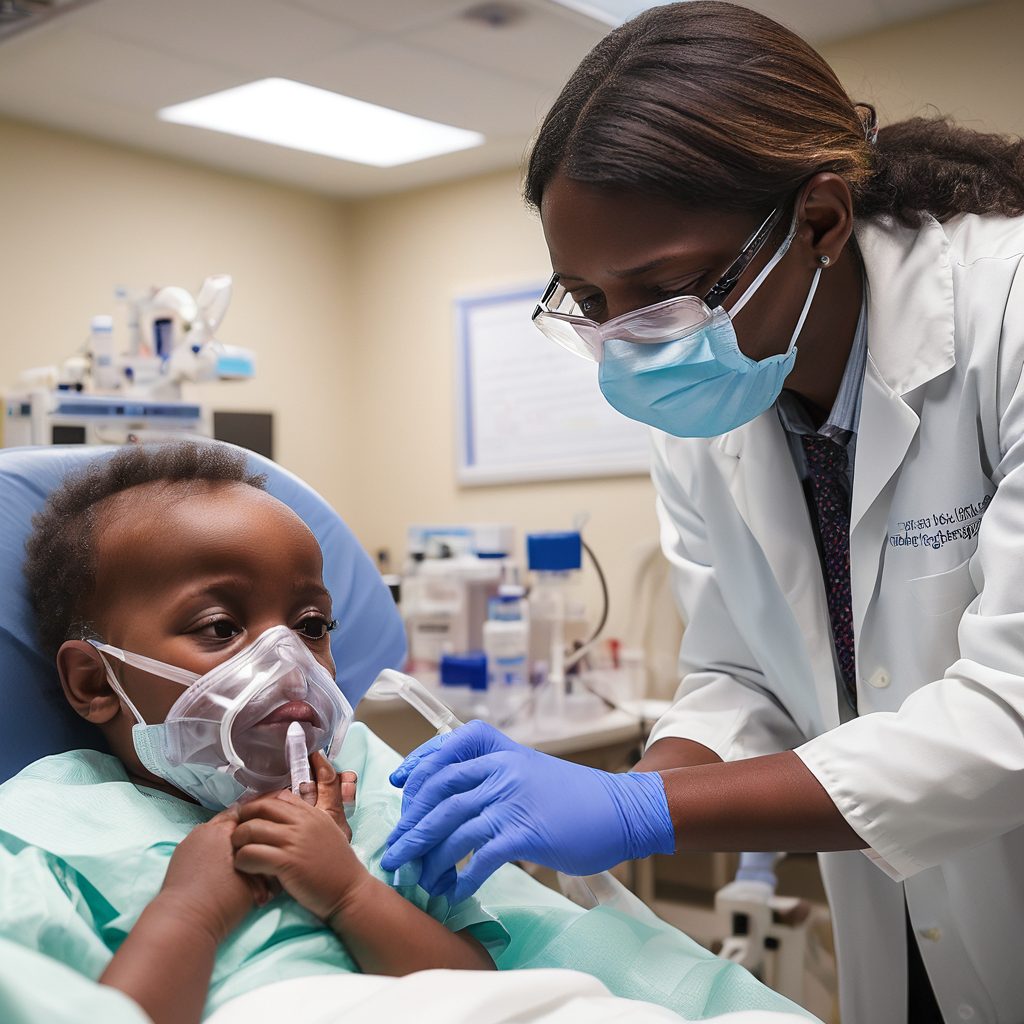
After 44 long days in the hospital, 12-year-old Kendric Cromer finally left Children’s National Hospital, marking a significant milestone in his battle against sickle cell disease. Wearing a T-shirt and cap from his favorite anime series, “Naruto,” and wheeled out by hospital staff cheering and celebrating, Kendric had just become the first patient to receive a newly approved gene therapy for sickle cell disease. This revolutionary treatment holds the promise of freeing him from the painful grip of a condition that had stolen much of his childhood.
“I thought I would have sickle cell for the rest of my life,” Kendric said, still processing the reality of his recovery. For years, the disease had kept him from enjoying simple pleasures like playing basketball or riding a bike, as even slight exertion could trigger agonizing pain and frequent hospitalizations.
Yet, despite the celebratory atmosphere, Kendric’s parents, Deborah and Keith, were still grappling with the intense challenges they faced during his hospital stay.
Nothing had truly prepared them for the journey. Not the in-depth discussions with doctors, not their diligent reading of medical literature, not even the extensive 13-page consent form that outlined potential risks like organ damage and death could have made them ready for what Kendric would endure.
Sickle cell disease affects around 100,000 people in the U.S., with roughly 20,000 facing the most severe form. For these individuals, gene therapy may be their only hope for a normal life. The disease, caused by a mutation in hemoglobin genes, leads to red blood cells that assume a crescent shape, blocking blood flow, causing excruciating pain, and damaging organs. For many, there had been no way out.
That changed in December when the Food and Drug Administration approved two groundbreaking therapies: Bluebird Bio’s $3.1 million treatment and Vertex Pharmaceuticals’ $2.2 million option. For families like the Cromers, this offered a life-altering opportunity—if their insurance covered the cost.
Kendric’s insurer approved the treatment with minimal complications, making him Bluebird’s first commercial sickle cell patient. But while the Cromers were fortunate in this regard, the reality of the treatment’s toll often goes unspoken. Families are so desperate for a cure that the arduous process can be overlooked in their hopes for recovery.
As Dr. Akshay Sharma of St. Jude Children’s Research Hospital noted, “Even though I say ‘this is a dangerous therapy, high risk, you could die,’ no one hears that. All they hear is ‘I could be cured.’”
Kendric’s treatment journey began on May 1, when doctors extracted stem cells from his bone marrow. These were sent to a facility in New Jersey, where new, healthy hemoglobin genes were added. By September, the genetically modified cells were ready to be reintroduced into Kendric’s body.
On September 3, Kendric was admitted to the hospital for the final phase of his therapy. Intense chemotherapy followed, designed to clear out his bone marrow and make way for the healthy, treated cells. The side effects were brutal. By mid-September, Kendric suffered from mucositis, a painful inflammation that led to severe sores in his mouth and intestinal tract. His tongue swelled so much that he could barely speak, and eating became impossible. For days, Kendric communicated with his parents using a stuffed dog with bells around its neck, shaking it to get their attention and writing notes to describe his needs.
The agony didn’t end there. Kendric’s hands and feet swelled and burned, his skin darkened and peeled, and his hair fell out. His pain, which he described as an 8 or 9 on a 10-point scale, was only numbed by high doses of morphine that left him in a stupor.
But slowly, Kendric began to heal. The swelling in his tongue subsided, and he regained the ability to talk. His journey to recovery wasn’t complete, though. Blood transfusions had left him with excess iron, which he will need to have removed through regular bloodletting sessions to prevent damage to his organs. Additionally, the chemotherapy left him with a weakened immune system, meaning Kendric will need to be re-immunized with childhood vaccines and avoid crowds for several months.
Despite the grueling process, Kendric’s parents are hopeful. “These kids are walking miracles,” his mother, Deborah, said. His father, Keith, echoed this sentiment, acknowledging that learning to live a normal, healthy life would be a new challenge for their son.
Kendric is also beginning to feel the transformation. “I haven’t felt all the amazingness yet,” he said. “But I am slowly starting to feel better.” One night, before his discharge, as they watched “Dancing with the Stars” on TV, Kendric turned to his mother and said, “Mommy, let’s dance.” They danced together in his hospital room—something that would have triggered a pain crisis in the past, but now left him feeling great.
“I felt great,” Kendric said with a smile. “Apparently, I’m a good dancer.”
Come and join us for Enhancing Speed & Quality in Cell & Gene Therapy Manufacturing for the DACH Region on 12th-13th February 2025 in Switzerland! This event will bring together leading experts and industry professionals to explore cutting-edge advancements in cell and gene therapy (CGT) manufacturing. You’ll have the opportunity to dive into breakthrough innovations like bioreactors, in-vivo cell engineering, and 3D cultures, while addressing challenges around regulatory compliance, scalability, and quality control. With over 200 attendees, 30+ expert speakers, and interactive panels, this summit is the premier platform for connecting with key stakeholders, gaining actionable insights, and driving the future of CGT manufacturing in the DACH region. Don’t miss your chance to be part of this transformative journey—see you there! Find out more at: https://imapac.com/events/cell-and-gene-therapy-manufacturing-summit/
Source: https://www.nytimes.com/2024/10/21/health/sickle-cell-disease-gene-therapy-patient.html